Well thermodynamics is actually a part of your daily routine. Thermodynamics has a Greek origin, which basically suggests the relation between Thermal energy and Mechanical Power. This is the science which studies the whole heat transfer process which involves all the characteristics such as the change in temperature, pressure, and volume. The whole refrigeration and heat pump cycle is completely based on thermodynamics.
Thermodynamics actually refers to the removal of heat from one object or substance, and then transferring it to the other, as we already know that the heat always flows from the hot body to the colder body.
Heat Pump Thermodynamics
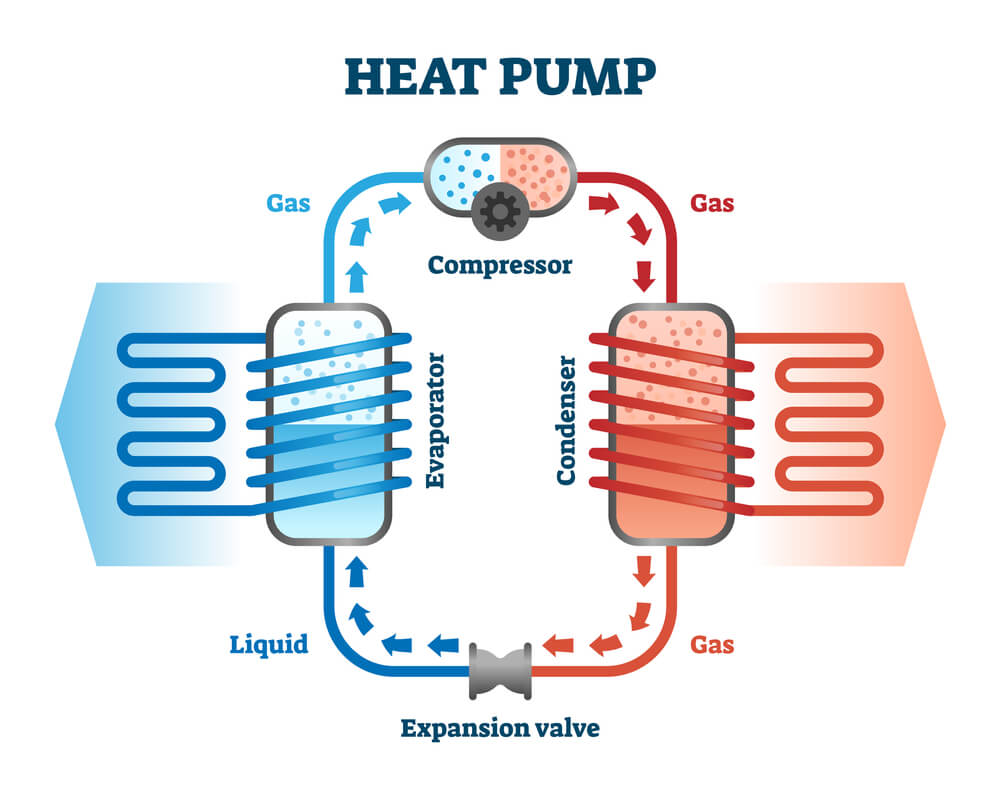
All the heat pumps, air conditioners, and refrigerators utilize heat transfer from cold to hot. Basically they use the heat engines that run backward. Here we are saying backward instead of reverse, because apart from Carnot engines, every other heat engine usually runs backwards, and they cannot be reversed. In these cases heat transfer occurs from a cold reservoir Qc into a hot one. This requires work input “W”, which is also converted to heat transfer process. Thus the heat transfer to the hot reservoir is Qh = Qc + W. (remember that Qh, Qc, and W are positive, with their directions indicated on schematics rather than by sign.)
A heat pump’s mission is to transfer heat Qh to occur into a warm environment, such as in any home in winter. The sole mission of air conditioners and refrigerators is for heat transfer Qc to occur from a cool environment, such as chilling a room or keeping food at lower temperatures than the environment. In fact, a heat pump can be used for both purposes, that is to heat and cool any space. It is very important to have an air conditioner and a heating unit all in one.
In these heat exchanges, the transfer can occur through three different processes, which are used by the refrigeration industry or have an impact on its efficiency, these processes include:
• Convection: The convection is basically the most present process in the refrigeration equipment you are dealing with. It usually occurs mostly in the fluids (Liquids and Gas). It is the outcome of a fluid circulation, which may happen either naturally, due to the differences in the temperature of the fluid or may be in the forced way. The heat exchange that happens on the evaporator and the condenser are the examples of convection.
• Conduction: well, the conduction usually happens between two objects that have somewhat different temperatures from each other, or it may also happen in only one object, but it always occurs from the hottest to the coldest area. It is generally related to the thermal conduction of every material. Regarding this process, it is very vital to remember that what defines a heat insulator is its low thermal conductivity, which is considered very essential for an efficient cooling system. For example, all those materials such as polyurethane that are used to insulate cabinets, keeping the internal temperature of the refrigerator lower than the external environment.
The irradiation is not directly connected to refrigeration but has an impact on the performance of the equipment. It occurs through electromagnetic waves, particularly infrared radiation, even without direct contact between bodies or substances. An example is the warming of the Earth by the sun, where there is no direct contact, but there is heat transfer.
• Radiation: Radiation is very important in order to keep refrigeration equipment away from any types of heat sources, so that the heat does not affect its performance in any way.
The Laws of Thermodynamics
This is the law that allows the definition of temperature scales, such as those expressed in degrees Celsius, Fahrenheit or Kelvin
There are two law of thermodynamics:
First law of Thermodynamics:
The first law of thermodynamics basically says that between any two equilibrium states, the change in internal energy is directly equal to the difference of the heat transfer into the system and the work done by the system. This law totally refers to modern refrigerators because of the reason that it defines that it is possible to raise the temperature of a system either by adding heat (thermal energy) or by doing work on it because it will produce heat that way.
Second Law of thermodynamics:
There are actually three different ways by which you can express this law, these ways were developed by scientists who realized the need to highlight certain characteristics, these ways are:
- It is impossible to remove thermal energy from a system at a certain temperature and convert that energy to mechanical work without any modification in the system or its surroundings. (Statement of Kelvin)
- There is no process where the only effect of thermal energy is to transfer energy from a cold body to a hot body. (Clausius Statement)
- It is impossible for a thermal machine, operating in cycles, to have the sole effect of extracting heat from a reservoir and performing an integral work of that amount of energy. (Kelvin-Planck’s statement).
The Refrigeration Cycle Thermodynamics
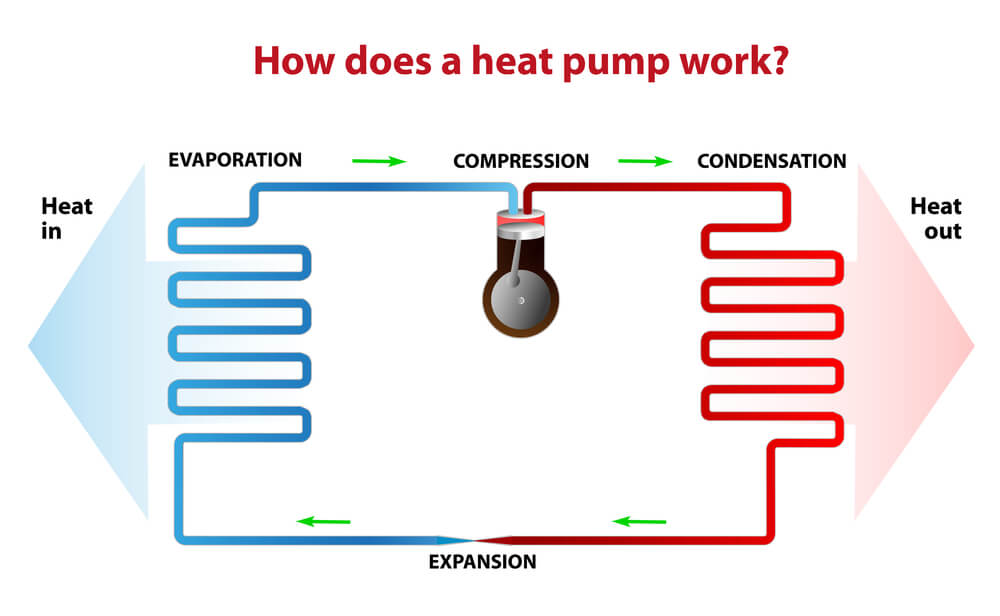
Consider it very important to know that conventional refrigerators usually work by following the principles of the mechanical steam compression cycle. But first you must know what it actually means? First of all, we need to know that this cycle is totally based on the process of changing the physical state of the refrigerant fluid that is from liquid to gas and from gas to liquid state. These substances become liquid which means condensation process at a high pressure and becomes gas which means evaporation at a very low pressure.
Whereas the cold in cooling systems occurs due to the change of state of this liquid refrigerant fluid to gas. This whole process totally depends on the work that is done by the compressor that uses mechanical energy to compress the refrigerant fluid from the evaporator in the gas form. With this compression process, the pressure and temperature of the refrigerant fluid increases in this way. When this gas enters the condenser, the refrigerant transfers the heat to the outer environment, which then causes its temperature to decrease and condensation occurs in this step, which is the process of changing this gas into liquid state.
After this point, the refrigerant fluid passes through the control element capillary tube or expansion valve, which, by narrowing the passage, slows the speed on the evaporator, which then causes its pressure to decrease. The refrigerant fluid arrives in the liquid state and under low pressure to the evaporator, during which it changes its phase again, from liquid to gas. When you change phase, it absorbs the heat present in the conditioned items in the refrigerator case and returns to the compressor, restarting the refrigeration cycle.
The Refrigeration Cycle
Your air conditioner always works with the thermodynamic cycle which is also called the refrigeration cycle. This process is done by changing the pressure and state of the refrigerant to absorb or release heat. The refrigerant which is also known as coolant then absorbs heat that is inside of your home and then pumps it outside of the home. Most of the air conditioners are air-source, split systems which means that there is only a single unit inside and the other unit is outside, which is why it is known as a split system.
The air-source part usually refers to the place where the thermal energy is thrown in the outside air. There are also other potential places from where the heat can be transferred, such as water or ground, they are also known as water-source, or ground-source systems. The inside unit is normally inside the house anywhere, it can be in the attic, basement, closet, or crawl space. The outside unit is normally located on the side or back of the building. All other kinds of air conditioning systems, such as ground-source and water-source, also follow the refrigeration cycle, but some of the specifics, such as location and parts may differ from each other.
Below are the basic parts of the refrigeration cycle it is exactly the same process that your refrigerator uses in order to keep the food cold:
Air flows over the indoor coils, which contain extremely cold refrigerant
At the time when air flows over the cold coils, all the heat from the air will get transferred to the refrigerant that is inside the coils. After this air flows over the coils, it will obviously get cold, and normally it drops down at 20 degrees. This process basically follows the 2nd law of thermodynamics that we have already discussed earlier, which states that heat naturally flows from a warmer body to a cooler body. After the refrigerant absorbs this heat, its state changes from a liquid to gas. This warmer refrigerant gas then gets transferred to the compressor which is step 2 in the refrigeration cycle.
Warmer, vaporized refrigerant gets compressed (pressurized) to a hot temperature
Even after the refrigerant has absorbed heat from the indoor air, it is still fairly cool. The still cool, but warmer vaporized gas enters the compressor (located in the outside unit) to increase its pressure and temperature. We increase the temperature of the refrigerant because it has to be warmer than the outdoor air. Here the 2nd law of thermodynamics works which says that the heat flows from warmer to cooler bodies. In case the refrigerant is 120 degrees, and the outdoor air is 90 degrees, the outdoor air will be cooler, which means that the heat from the refrigerant will flow in the direction that exactly we require, that is the outside. For instance if the temperature outside is 120 degrees, the compressor will have to work harder in order to increase the temperature of the refrigerant to a higher temperature. So, after the refrigerant’s temperature is increased above that of the outdoor air’s temperature, it will flow into another set of coils, which is known as the condenser coils that are also located outside.
Very hot refrigerant flows into condenser coils where it loses heat to the outdoor air

As at this point the refrigerant has been compressed which is also known as the process of pressurization, this will now be hotter than the outdoor air. A condenser fan will blow hot air that uses outdoor air over the hotter outdoor condenser coils. As the outdoor air flows over the outdoor coils, heat will be removed from the refrigerant and will be released into the outdoor air. Once again, this is because of the 2nd law of thermodynamics. After the refrigerant loses the thermal energy to the outdoor air, it will condense back into a liquid and will get pumped back inside.
The still warm refrigerant from the outdoor unit needs to get cold
After the refrigerant leaves your outdoor condenser unit, its temperature will still be very high. The refrigerant’s temperature will have to drop significantly before it can absorb more heat from the indoor air. The metering device, which is usually a thermostatic expansion valve, is a special device that will depressurize the refrigerant, which can cause a drop in temperature. It will be done by expanding the refrigerant into a larger volume. The refrigerant always has to be colder than the indoor air in order to absorb heat. After the refrigerant gets cooled down, it flows back into the evaporator coils where it begins the refrigeration cycle again.
Hopefully, this helps you understand the basic workings of an air conditioner. The refrigeration cycle is basically the same for your freezer as well as refrigerator.

